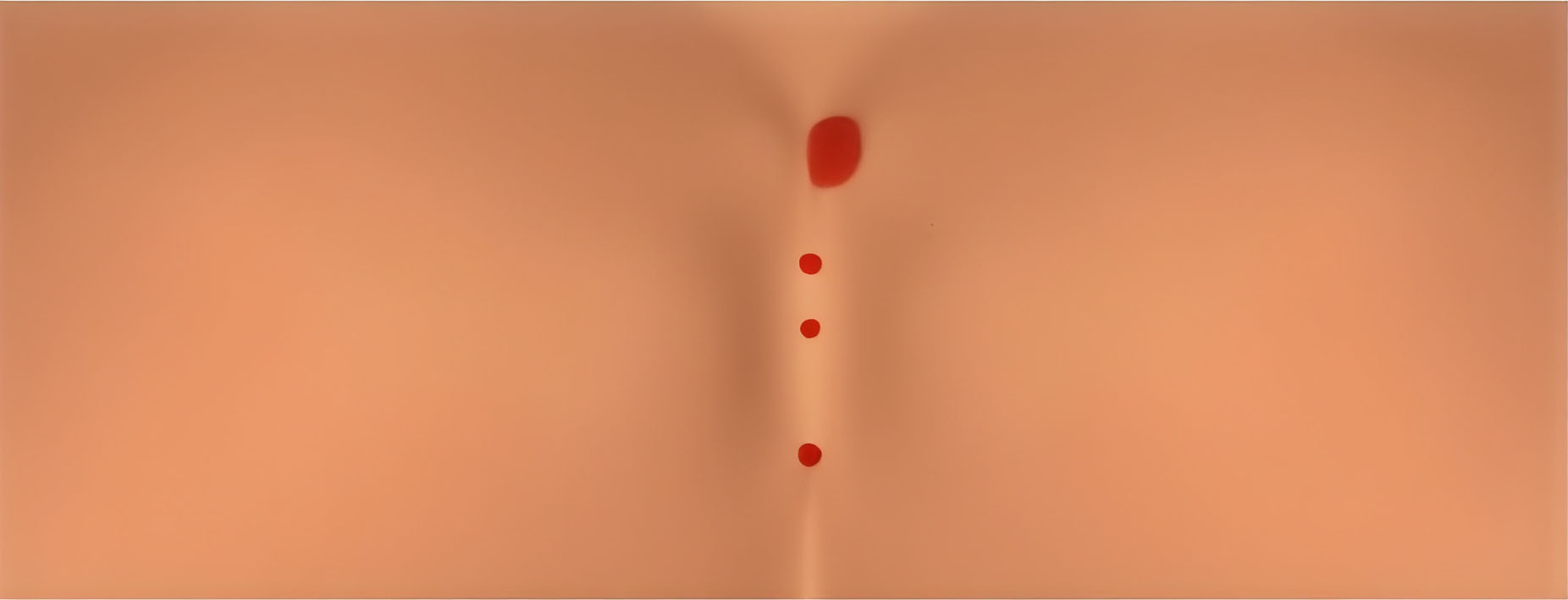
1. Disinfect, anaesthetize, core with biopsy punch or trephine.
Pilonidal Sinus, known for recurrence and discomfort, is conventionally treated with invasive surgical procedures. This standard of care involves extensive incisions and prolonged recovery, highlighting the necessity for more efficient and minimally invasive treatment alternatives.
Our approach integrates a minimally invasive procedure in combination with the application of RD2 Ver.02 autologous blood clot.


Pilonidal Sinus pose recurrent treatment challenges, necessitating effective, minimally invasive solutions. Our innovative approach combines a modified procedure with autologous blood clot, offering promising outcomes for patients suffering from this condition.
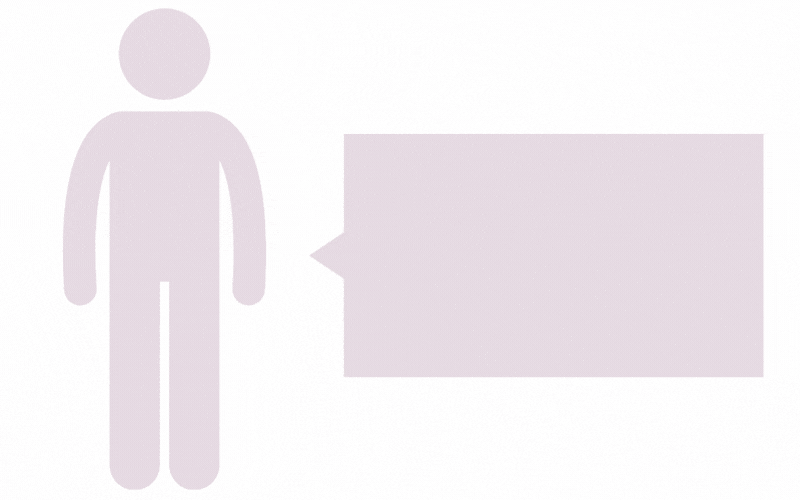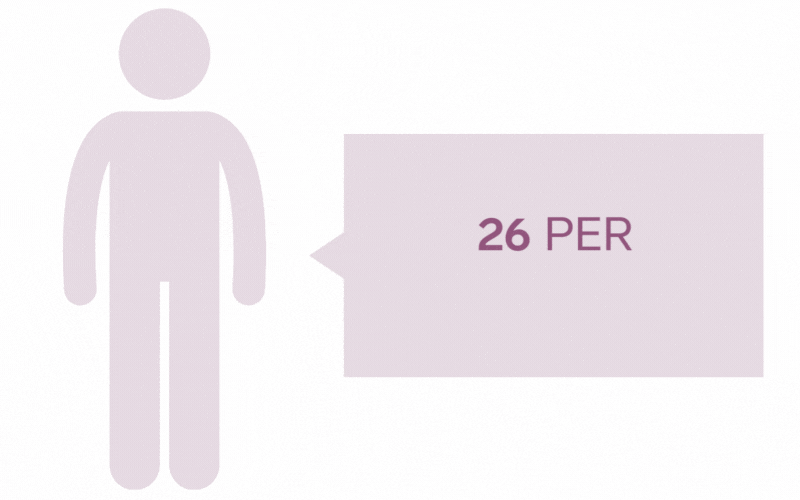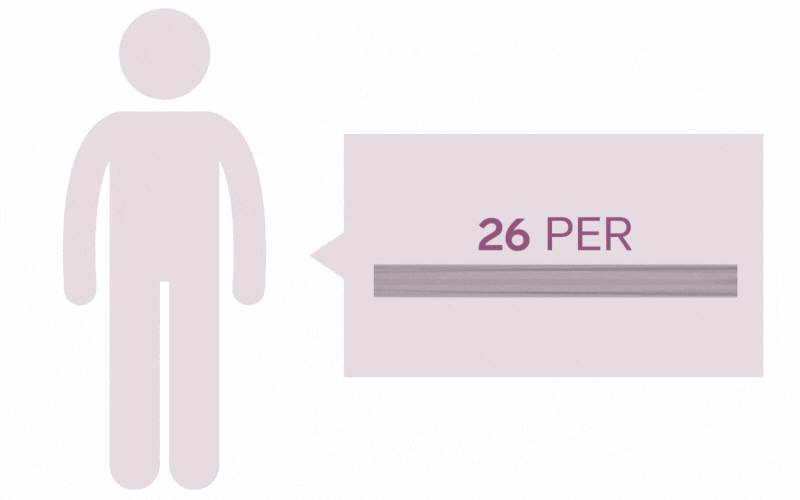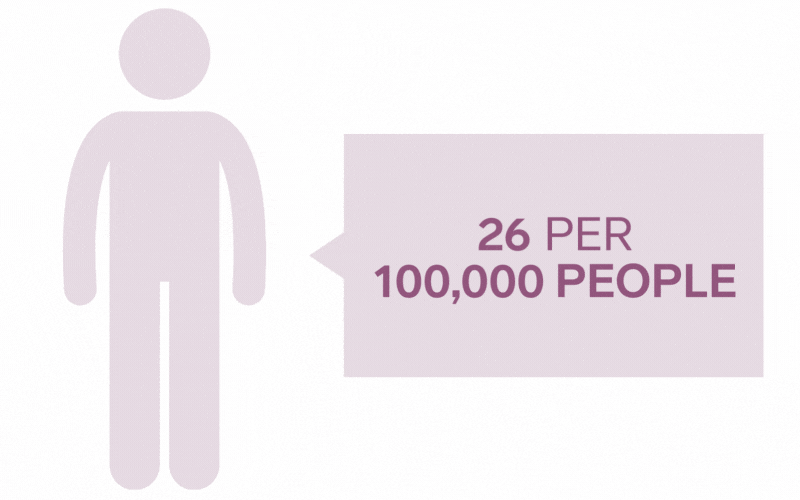
Pilonidal Sinus are a common disease affecting up to five percent of the population. The incidence of pilonidal disease is estimated to be 26 per 100,000 people and affects men 2.2 times more than women. It is estimated that pilonidal disease affects approximately 70,000 people in the United States annually.
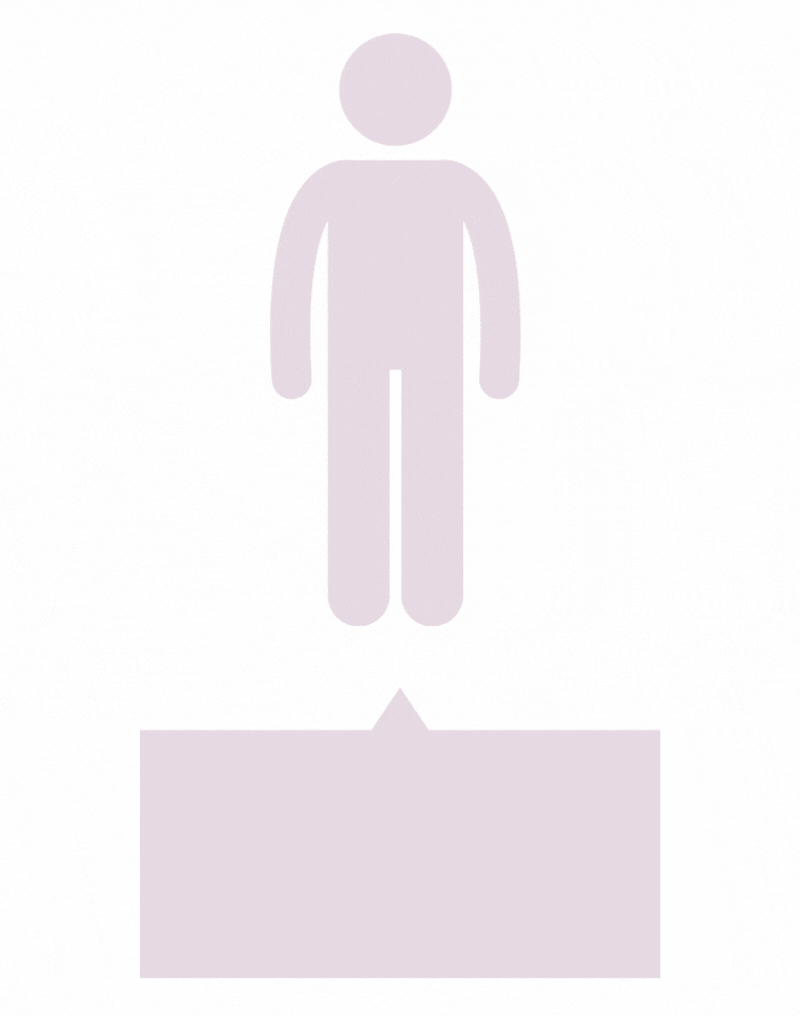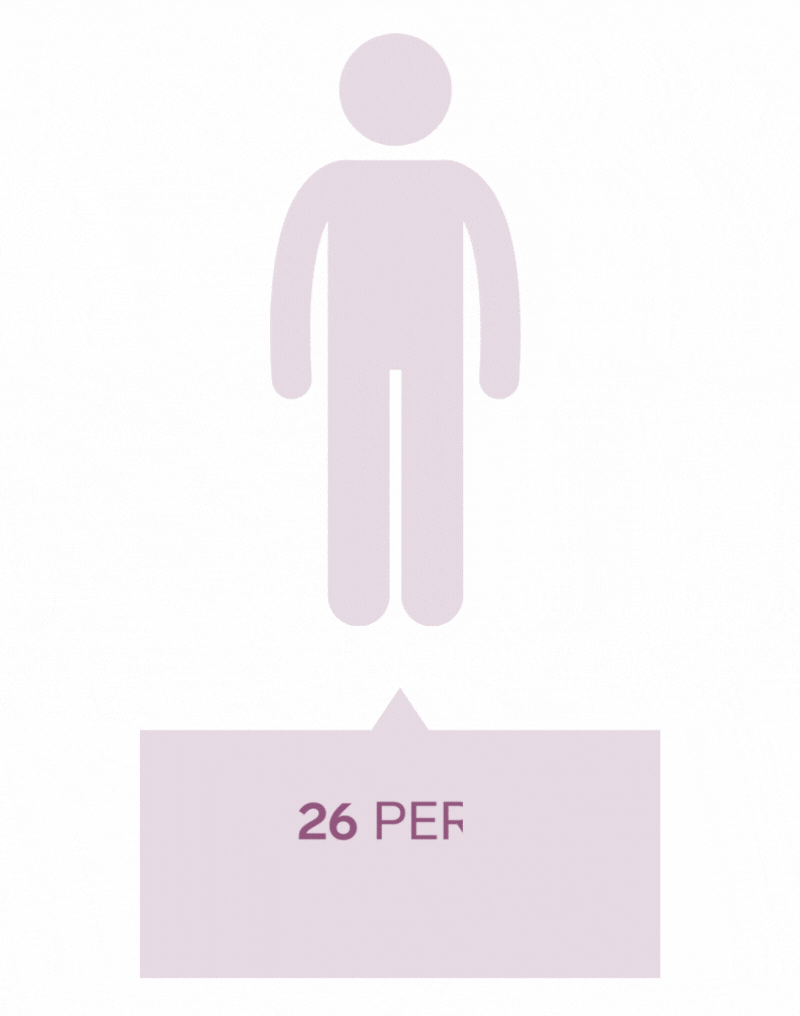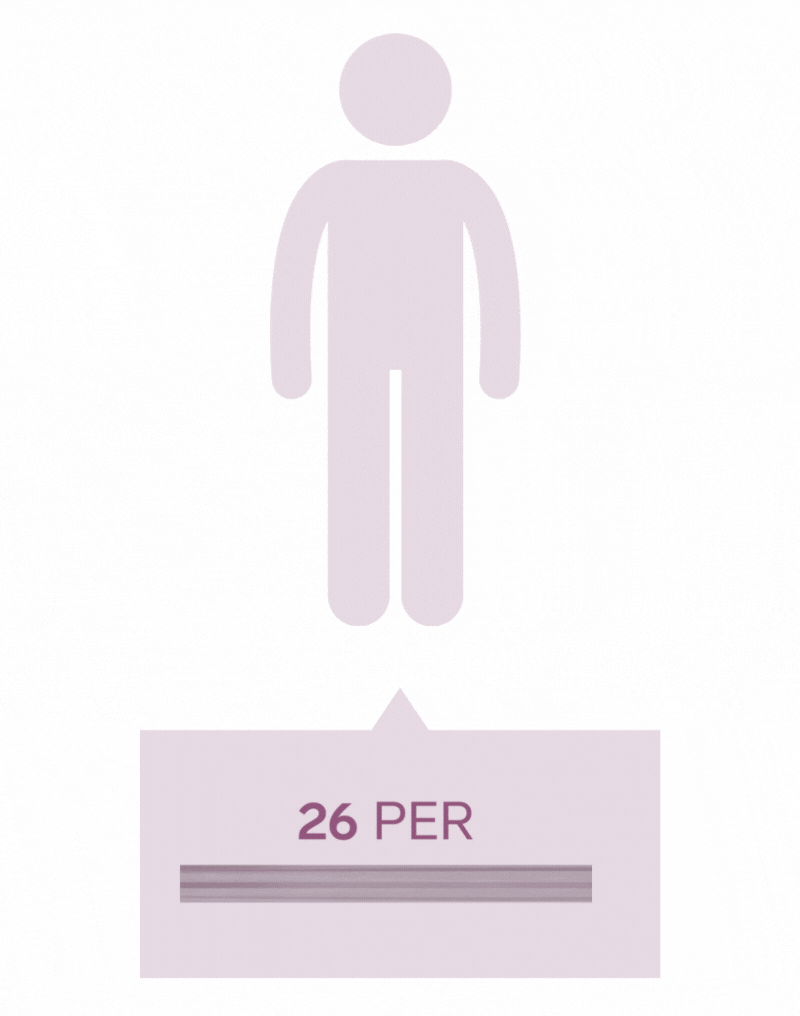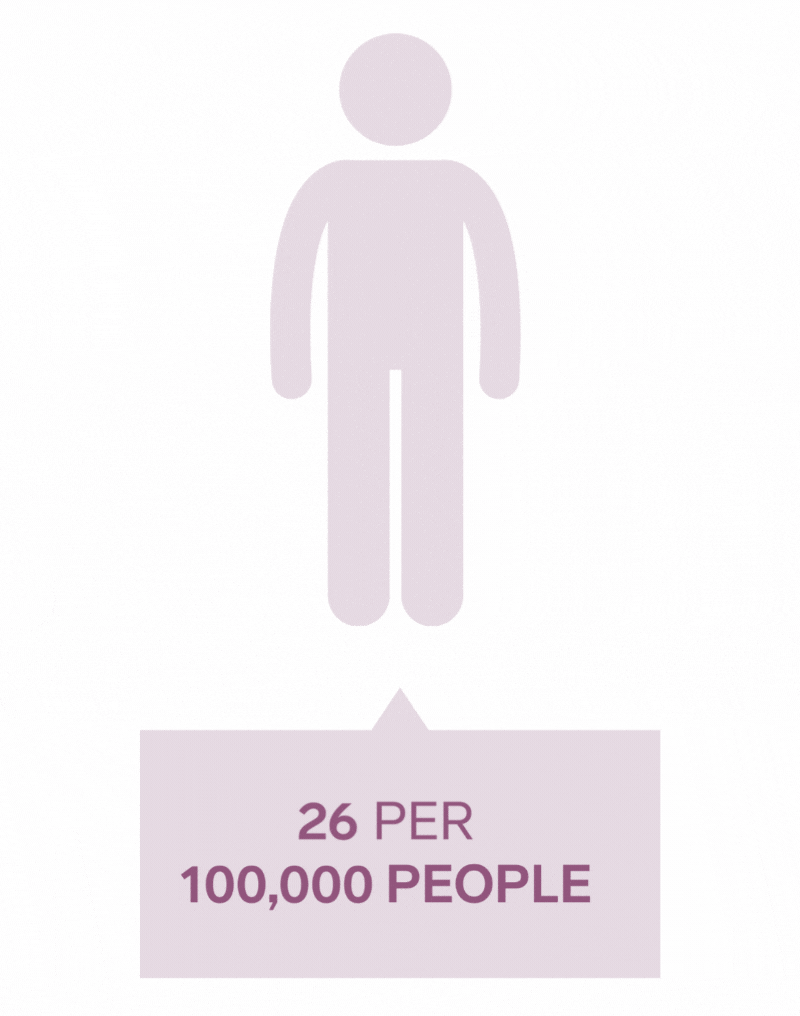
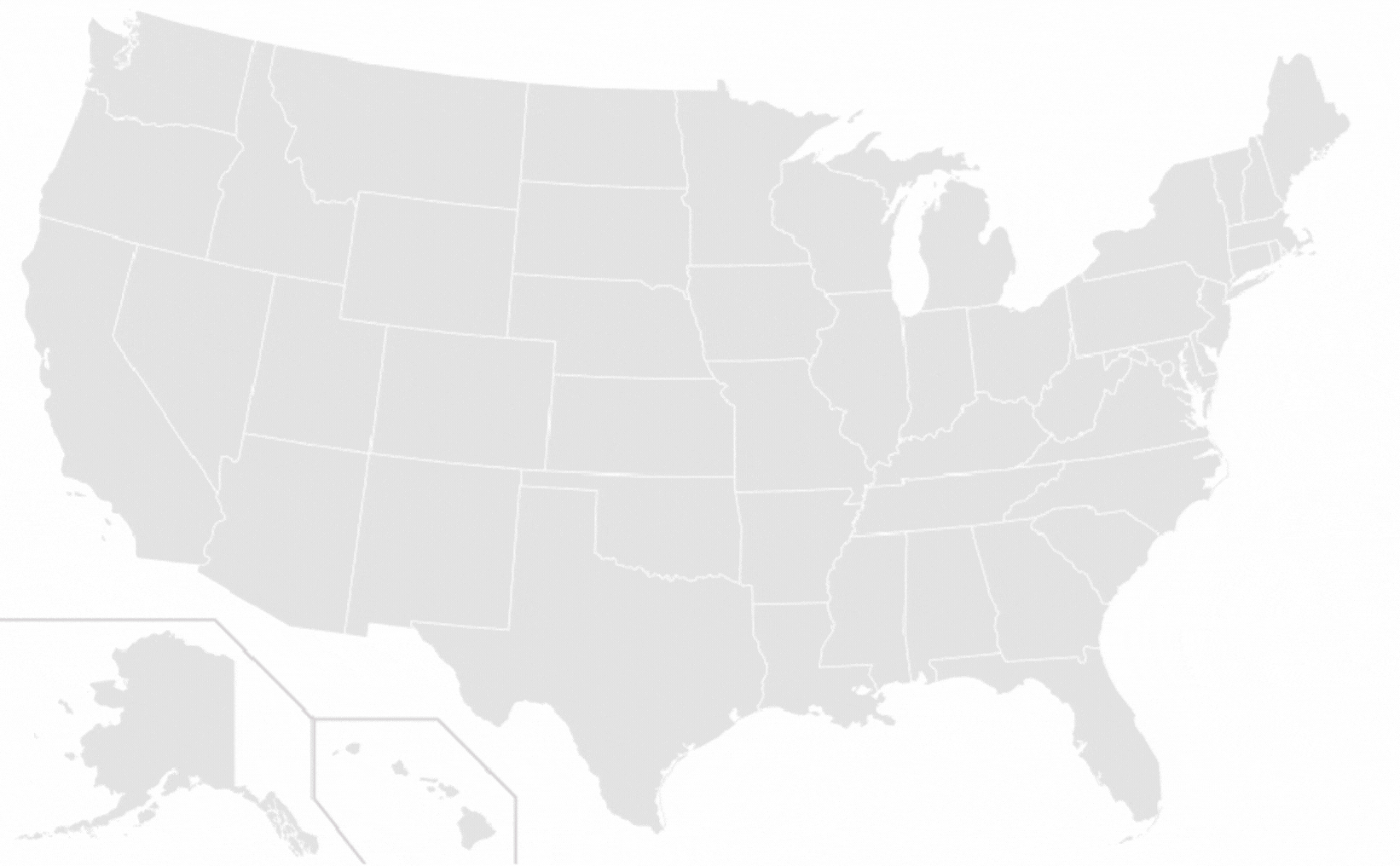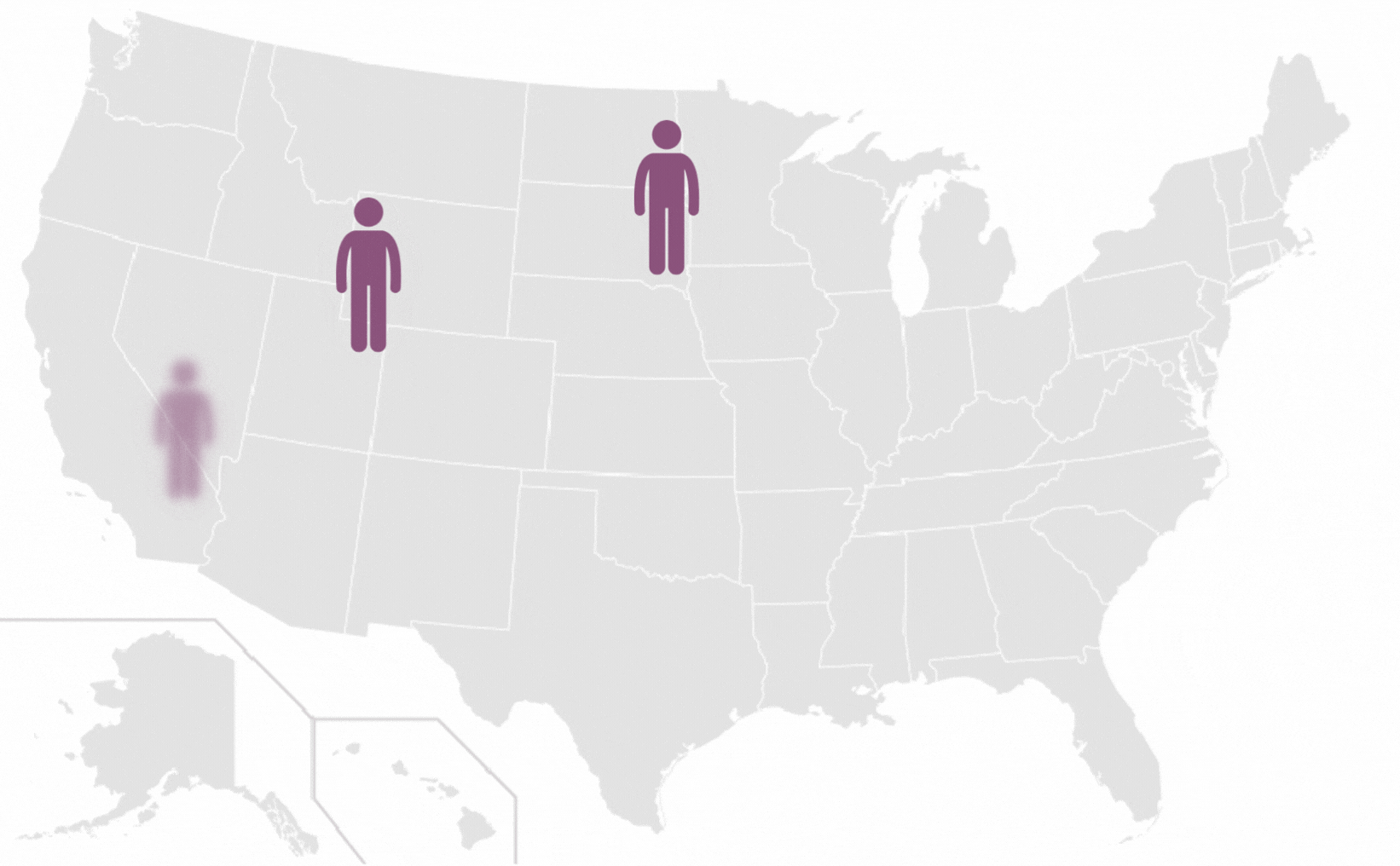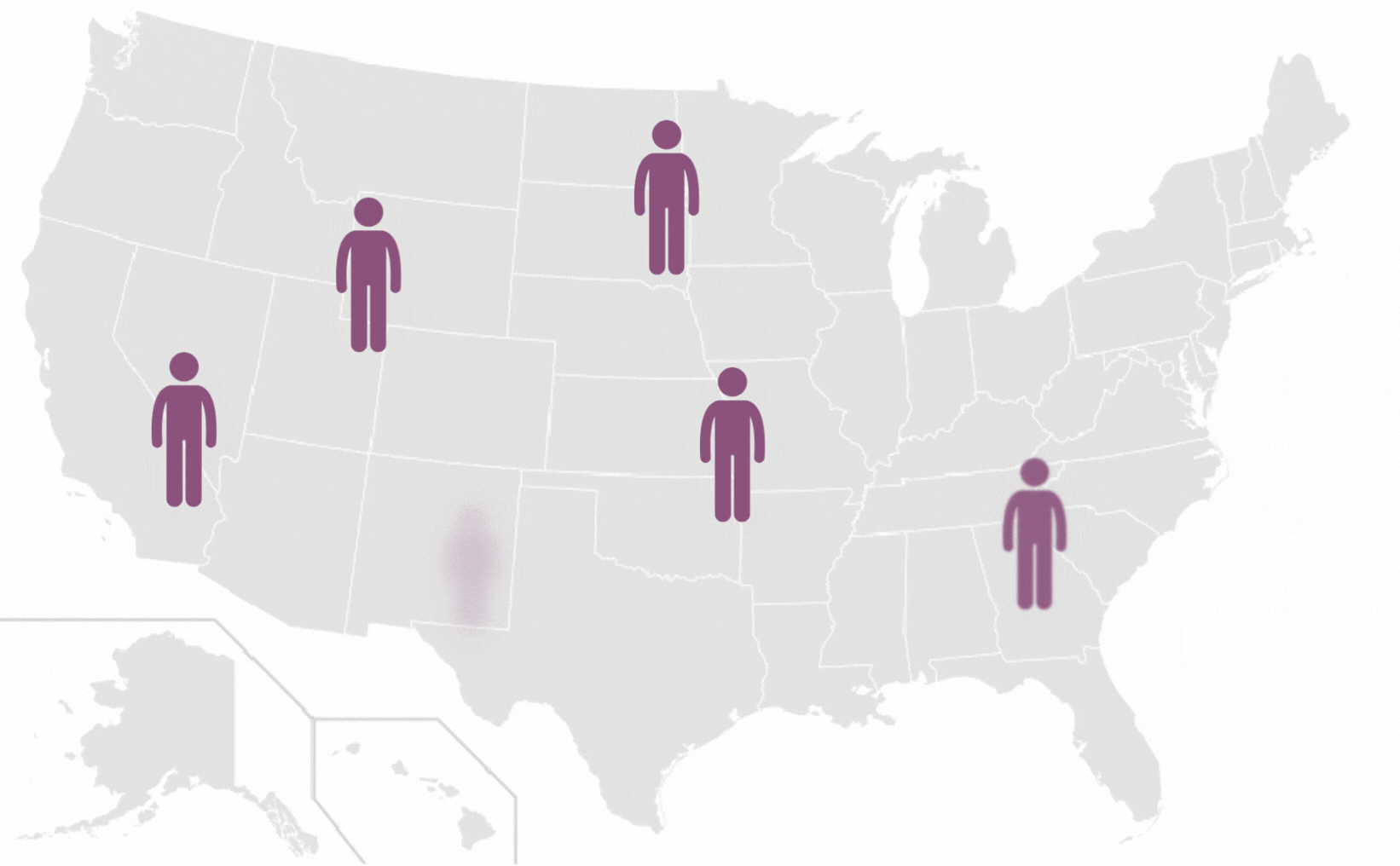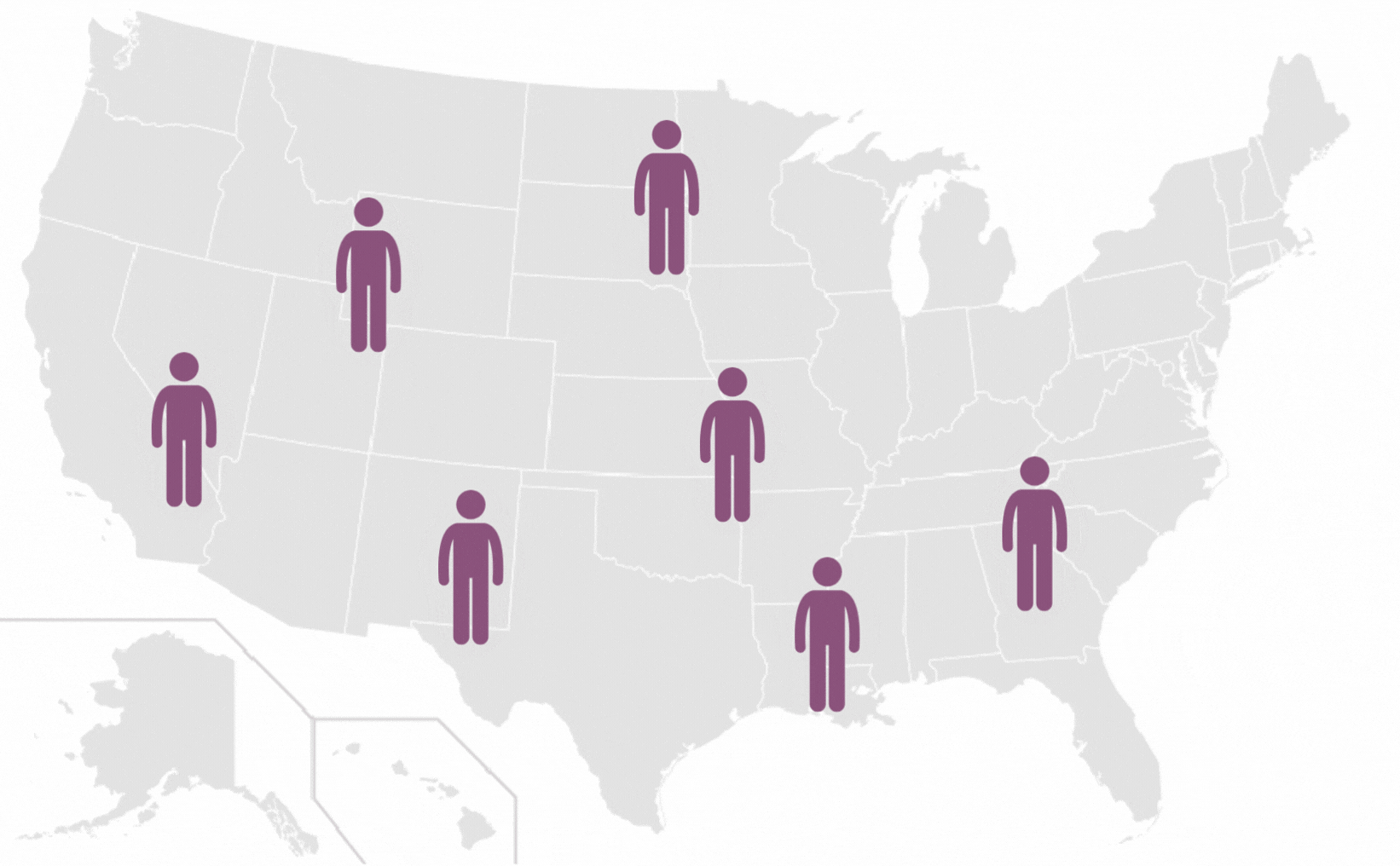
*Each icon represents 10 000 people


1. Disinfect, anaesthetize, core with biopsy punch or trephine.
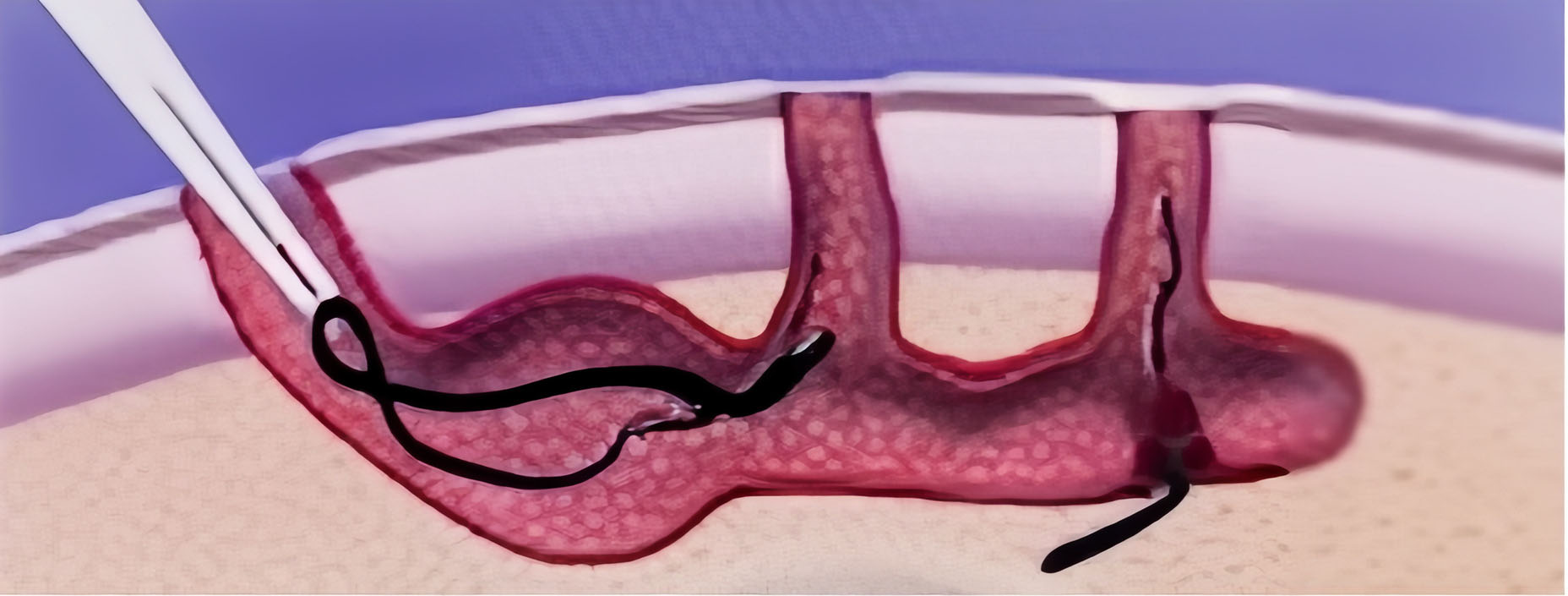
2. Core and debride sinus tracts while removing hair and debris.
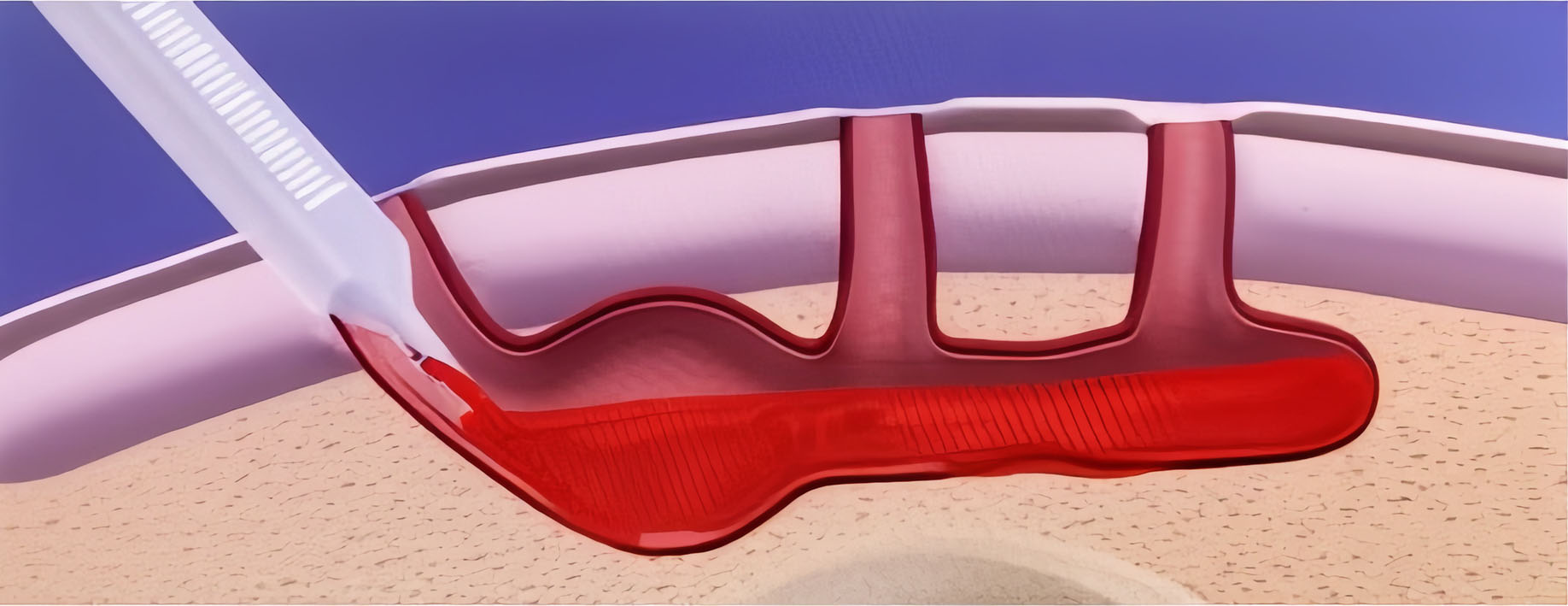
3. Irrigate, apply ActiGraft, dress for drainage
Prospective pilot study conducted at Sheba Medical Center evaluated the Safety and Efficacy of RD2 Ver.02 with Minimally Invasive Pit Excision Procedure in Pilonidal Sinus Disease.

AT 6 Months

RD2 Ver.02, or ActiGraft, is suitable for pilonidal disease in countries with local regulatory approval, providing an innovative solution for this chronic skin infection.